Alexander Fleming’s discovery of penicillin in the late 1920s revolutionized the treatment of infectious diseases. But the increasing prevalence of antibiotic resistance and the emergence of multidrug-resistant ‘superbugs’ shows the limitations of what was once considered a wonder drug.
In his 2018 TED talk Alexander Belcredi, MBA, the Chief Executive Officer of PhagoMed Biopharma, a German biotechnology company developing phage-based pharmaceuticals to treat bacterial infections, highlighted the threat posed by the growing antibiotic crisis, when he said that antibiotic drug resistance today kills, on average, over 700,000 people every year. Failing to solve this problem means that we re-enter an apocalyptic pre-antibiotic era, with an estimated 10 million people dying each year by 2050.
Belcredi also suggested a solution to the problem: “[Bacteriophages] are the superheroes that we have been waiting for in our fight against multidrug-resistant infections.”
Bacteriophages: The Good Viruses
But what are bacteriophages? How might they be used to fight superbugs? And what are challenges that need to be overcome before they are used in clinical practice?
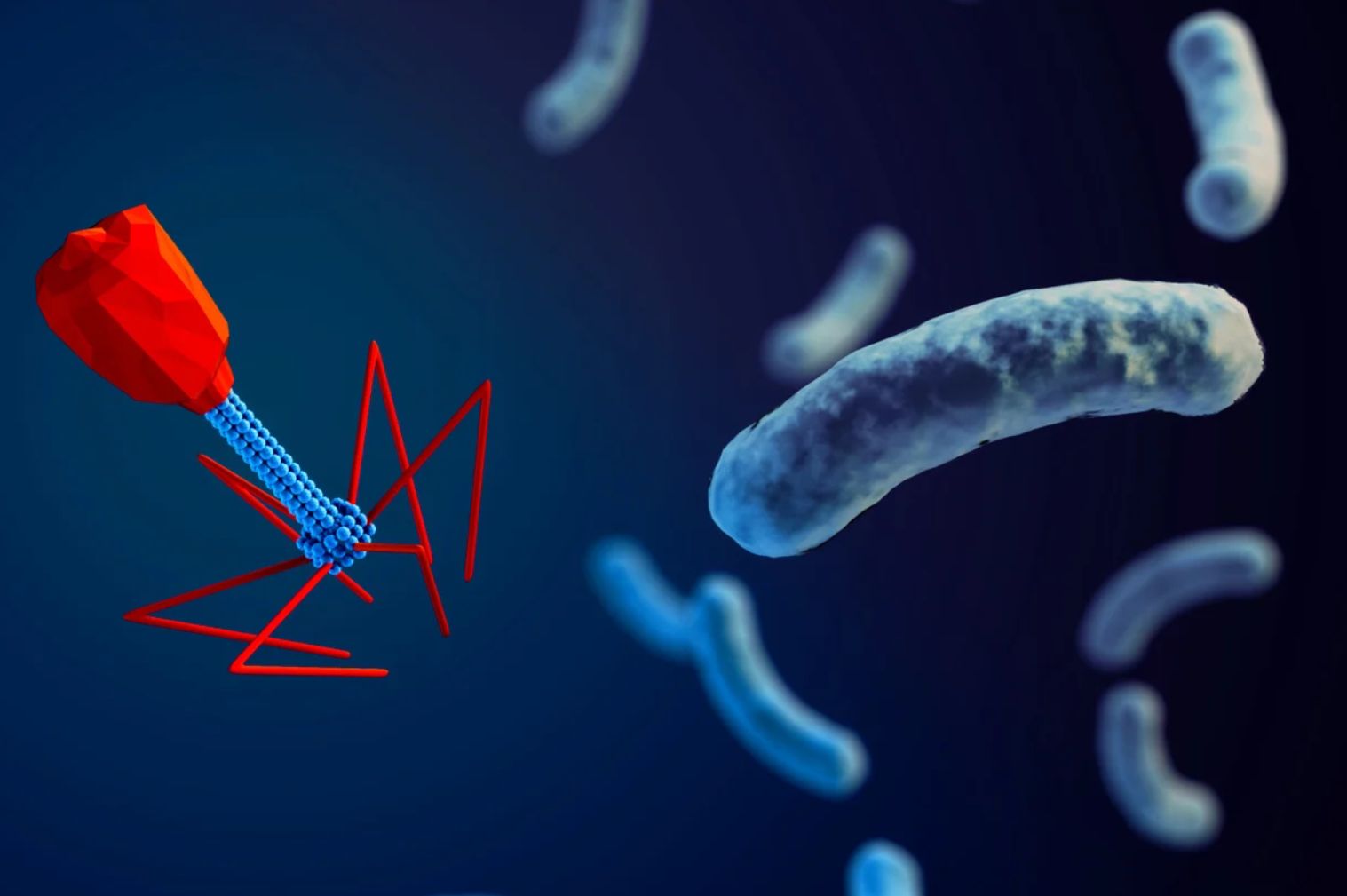
Bacteriophages are naturally occurring viruses that hunt, invade and kill specific, disease causing, bacteria.
In 1916 Felix d’Herelle, a French-Canadian microbiologist at the Pasteur Institute in Paris, France, was the first to develop and use phage therapies for typhoid and urinary-tract infections.
However, Fleming’s discovery significantly limited the interest in phage-therapy in Europe and the United States. As a result, they were forgotten by modern medical science until the rise of multidrug-resistance bacteria.
Similar to Antibiotics
Clinical Trials
Challenges
However, to become mainstream therapeutics, there are several hurdles that need to be overcome, including the limited stability of bacteriophages in solution. Furthermore, little attention has been given to the effect of formulation on phage therapy outcomes, especially since bacteriophages may need to be administered as cocktails to combat more than one bacterial target.
To be successful in meeting these and other challenges, drug developers are required to setup and execute more clinical studies. They will need to combine data from multiple sources for which modern eClinical systems may be helpful.